Compliance with the Sunshine Act is an obligation for MedTech companies, yet this sector faces recruitment challenges – from the ‘Great Resignation’ to quiet quitting – that are draining internal expertise.
Added to this, MedTech firms may not have sufficient internal capabilities to support transparency compliance and thus may take a more entrepreneurial approach. The MedTech sector includes 908 firms in the U.S.1 with revenues of $49.1 billion in 20222. With lean organizational structures, MedTech companies may have an inclination for a more flexible, innovative approach to compliance. One option is to outsource transparency reporting to an experienced partner, freeing up internal expertise year-round for core activities with greater strategic impact.
Over nine years have passed since the Sunshine Act (also known as the Open Payments Program) was enacted in 2013.3 While the first reports were submitted under the Act in 2014, there were no public-facing government actions or settlements until October 2020. Since then, three civil monetary penalties (CMPs) to the Department of Justice have been disclosed, totaling $2.2 million.4 All three involved the medical device manufacturing sector5 6 – possibly highlighting a need for external support in compliance.
Skillsets and capacities to look for in a partner
In reviewing their approach to transparency reporting, MedTech companies may wish to evaluate potential external partners based on six key skill sets and capacities:
- Availability of multiple transparency reporting specialists with extensive experience of Sunshine Act compliance, and understanding of legal developments in medical device markets worldwide. This can offer cost-effective access for MedTech companies to compliance experts, lawyers, and data analysts, without needing to employ them full-time.
- Labor resources that are tailored and cost-effectively priced to meet MedTech reporting needs, making best use of each expert’s skills, rather than relying on a few internal employees to ‘wear many hats.’
- An extensive global footprint enabling the vendor to scale geographically to meet language, legal, and time zone requirements.
- Flexibility to increase or decrease resourcing based on client needs and compliance timelines, including an option to add vendor personnel to supplement the client team.
- Latest technologies and best practices to ensure full compliance, including standardized, well-documented processes.
- Proven ability to manage all administrative tasks related to compliance.
Sunshine Act statistics (April 2022)
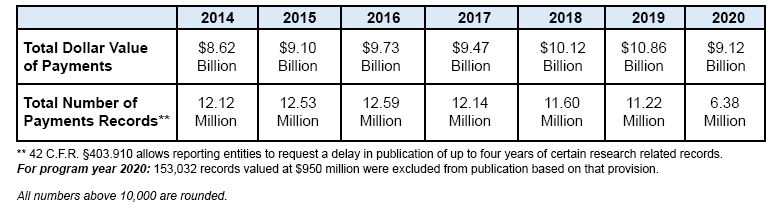
In program year 2020, $9.12 billion in publishable payments and ownership and investment interests were reported under the Sunshine Act (also known as the Open Payments program).9 These payments are comprised of 6.38 million payment records attributable to 487,152 physicians and 1,213 teaching hospitals. Since the program’s inception in fiscal year 2014, CMS has published more than 78 million records totaling more than $59 billion. Citing the Open Payments statute, the Department of Justice (DOJ) settled three cases of non-compliance with the Open Payments program.
The three civil monetary penalties totaled $2.2 million.
Summary of program year data 8
The Sunshine Act
The Sunshine Act – formally, the Physician Payments Sunshine Act (PPSA), or section 6002 of the Affordable Care Act of 201011 – requires medical product manufacturers to publicly report any payments to physicians or teaching hospitals.12 As written in Health Affairs in 2014, “These relationships can have many positive outcomes and — particularly in the context of consulting and research funding — are often a key component in the development of new drugs and devices. However, they can also create conflicts of interest and in some cases can blur the line between promotional activities and the conduct of medical research, training, and practice.”
The legislation applies to manufacturers of drugs, devices, biologics, and medical supplies covered by Medicare, Medicaid, or the Children's Health Insurance Program. Three categories of payments or other transfers of value to physicians must be disclosed:
- General payments (of transfers of value) such as meals, travel reimbursement, and consulting fees
- Investments such as stocks, licenses and royalties by physicians and close family members
- Payments for research, clinical trials, or other development activities; this covers research subject to a written agreement or a protocol.
These must be disclosed to the Centers for Medicare and Medicaid Services, and are posted on a public website. In 2021, disclosure was expanded to include five additional provider categories: physician assistants; nurse practitioners; clinical nurse specialists; certified registered nurse anesthetists and anesthesiologist assistants; and certified nurse-midwives. The ‘nature of payment’ categories were also updated to include debt forgiveness, long-term medical supply or device loan, and acquisitions.13
Reference
- https://www.ibisworld.com/industry-statistics/number-of-businesses/medical-device-manufacturing-united-states/
- https://www.ibisworld.com/united-states/market-research-reports/medical-device-manufacturing-industry/
- Centers for Medicare & Medicaid Services (CMS), HHS. Medicare, Medicaid, Children's Health Insurance Programs; transparency reports and reporting of physician ownership or investment interests. Final rule. Fed Regist. 2013 Feb 8;78(27):9457-528. PMID: 23476977.
- Centers for Medicare and Medicaid Services Fiscal Year 2021 Annual Report to Congress on the Open Payments Program (April 2022); https://www.cms.gov/files/document/open-payments-fy-2021-annual-report-congress.pdf
- https://www.paulhastings.com/insights/client-alerts/is-two-a-trend-recent-government-resolutions-hint-at-sunshine-act
- https://www.pietragallo.com/publications/the-physician-payments-sunshine-act-and-the-future-of-healthcare-transparency-part-2/
- https://jamanetwork.com/journals/jama/article-abstract/2798217
- https://www.statnews.com/2022/10/31/advanced-practice-clinicians-industry-funding/
- Centers for Medicare and Medicaid Services Fiscal Year 2021 Annual Report to Congress on the Open Payments Program (April 2022); https://www.cms.gov/files/document/open-payments-fy-2021-annual-report-congress.pdf
- https://www.cms.gov/files/document/open-payments-fy-2021-annual-report-congress.pdf
- https://www.govinfo.gov/content/pkg/PLAW-111publ148/pdf/PLAW-111publ148.pdf
- https://www.healthaffairs.org/do/10.1377/hpb20141002.272302/full/
- https://www.cms.gov/OpenPayments/Program-Participants/Reporting-Entities/Changes-for-Reporting-Entities

Learn how IQVIA MedTech can help
Contact us today for more information









