Specialized expertise and customized solutions across 14 therapeutic centers of excellence, including oncology, GI/NASH, pediatrics, neurology and rare diseases.
Advanced therapeutics medicinal products (AMTPs) offer transformative treatment approaches to patients in areas that were previously considered uncurable. CAR-T-cell therapies have shown impressive results in treating certain blood cancers and gene therapies like Zolgensma has proven long-term efficacy in babies suffering from spinal muscular atrophy (SMA)1. Innovation does not stop. In 2022, the EMA approved Biomarin’s gene therapy Roctavian for patients suffering from severe haemophilia A and 2023 might see the first-ever approval of Vertex & CRISPR Therapeutics’ exa-cel gene-editing treatment for sickle cell disease and transfusion-dependent beta thalassemia2.3.
This is the first instalment of our series of regular articles on developments in the space of cell, gene and RNA therapeutics, focusing on the global ATMP market developments and the innovation landscape.
ATMP market developments in 2022
Six novel advanced therapies were approved in 2022 – 4 gene therapies, 1 cell therapy and 1 RNA therapy. These products were approved for a range of indications, including one each for haemophilia A and B (Roctavian and Hemgenix respectively), which were the first gene therapy approvals for each of these conditions4,5. Additionally, the approval of Carvykti for refractory multiple myeloma brings the total number of approved CAR-T therapies to six6. Adstiladrin was also approved for an oncology indication – a form of bladder cancer7. One more gene therapy, Upstaza, was approved for AADC deficiency, and Amvuttra, the latest RNAi therapy was approved for hATTR amyloidosis8,9. We also saw CAR-T therapies expanding their indications and patient populations, with Yescarta and Breyanzi gaining approvals as second-line therapies in large B cell lymphoma.
Sales of advanced therapies (excluding COVID vaccines) continued their prolific growth in 2022. As figure 1 shows, the market reached $8.1 billion in 2022, with a 5-year CAGR of 60% since 2017. This is significantly higher than the top 10 therapy areas by value, including oncology (11%), immunology (17%) and antidiabetics (18%).
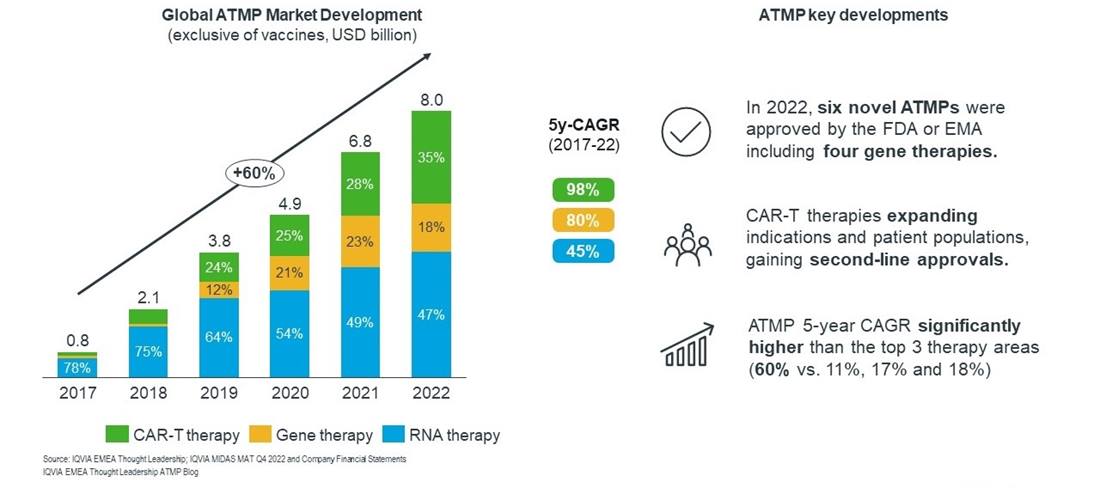
Nearly half of all advanced therapy market value in 2022 came from RNA therapies. However, within the RNA category nearly half of sales again were from Spinraza (nusinersen), a medication for SMA accounting for nearly $1.8 billion in total sales in 2022. SMA is a notable area of competition, with the gene therapy Zolgensma (onasemnogene abeparvovec-xioi) bringing in $1.4 billion in 2022, or 91% of gene therapy sales in our dataset. The SMA space became even more crowded after the launch of the small molecule therapy Evrysdi (risdiplam) which generated sales of around $1.2 billion.
Cell therapies comprised nearly one-third of all sales in 2022, with CAR-T therapies dominating within the category. With six CAR-Ts now launched, combined their sales were $2.6bn in 2022, with Yescarta (axicabtagene ciloleucel) being the lead product with $1.2bn in sales.
Overall, between 2021 and 2022 the market grew by $1.3bn, and although RNA may represent the largest segment, over 70% of the growth came from cell therapies, where in turn growth was driven by the CAR-T therapies. Cell therapies represented the highest growth rate of the three categories, with a 5-year CAGR of 98%. This impressive growth is likely to continue as the CAR-Ts face increased competition between themselves and other products, and as they seek to expand into more indications and receive approval for use earlier in the treatment journey. CAR-T therapies will also face some headwinds with the emergence of antibody-drug conjugates (ADCs) and bispecific antibodies in certain haematological cancer indications.
ATMP innovation landscape
In 2022, 767 next-generation biotherapeutics were in active development from discovery until regulatory submission and has grown significantly with a 20% CAGR since 201710. Cell therapies at 40% account for the largest share followed by gene and RNA therapies – exclusive of prophylactic vaccines – at 32% and 28% respectively (Figure 2).
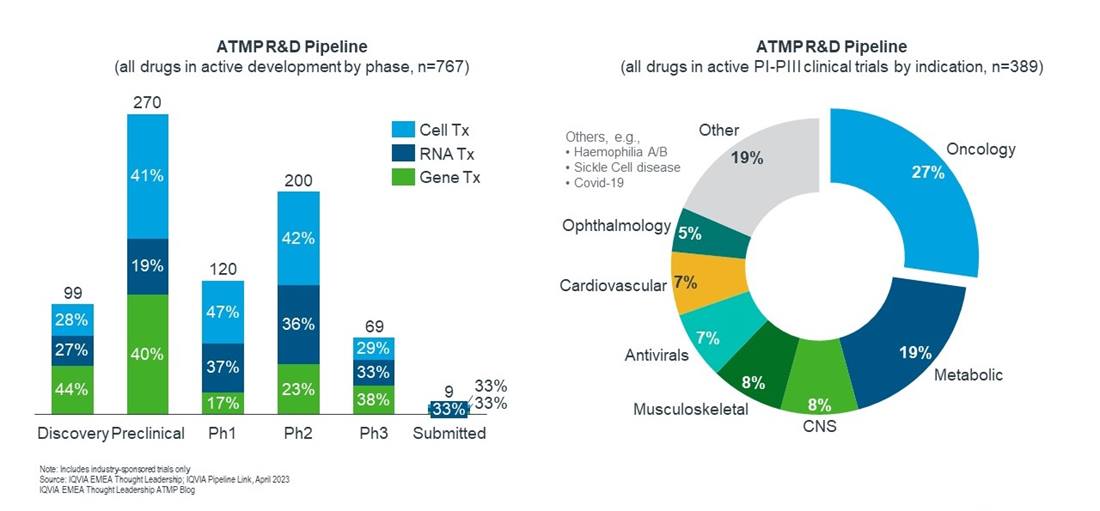
Oncology with $184 billion in sales was the largest therapeutic area and drove global sales growth in 202211. This high commercial potential makes oncology also an attractive area for ATMP innovators and makes up 27% of the late-stage clinical pipeline. Majority of products in active clinical development are CAR-T-cell therapies in haematological cancer indications. Followed by NK-cell therapies that offer the promise to overcome some challenges of CAR-Ts including lower manufacturing costs and fewer adverse events. The RNA companies BioNTech and Moderna are testing mRNA vaccines in combination with checkpoint inhibitors. Only recently, Moderna and Merck showed that such a combination reduced the recurrence of skin cancer by 44%12.
Comprising nearly one fifth of all ATMP drugs in the late-stage pipeline, metabolic diseases represent a high area of interest for R&D. Gene and RNA therapies are the major drivers in this therapy area, consisting of 40% and 54% of metabolic ATMP products. Particular indications of interest include non-alcoholic steatohepatitis (NASH), amyloidosis and diabetes. Interest is high for metabolic diseases, with Novo Nordisk recently entering a partnership with a biotech for four cell therapies for obesity and diabetes13.
The overall CNS pipeline has expanded by 31% in the past five years reflecting the high unmet need in that area and accounts for 8% of the ATMP clinical products14. RNA-based products are the leading platform whilst cell and gene therapies are in more advanced stages of clinical development. Examples of the latter include investigation products that aim to restore treatment benefits in late-stage Parkinson’s patients but progress to date has been erratic.
The year ahead
Innovation will not stop in 2023. According to the Alliance for Regenerative Medicine, there are at least 10 new cell and gene therapy products that could be approved in 2023 in the US and Europe. We have already seen Hemgenix approved in the EU (was approved in US in 2022), but the rest are novel products. This includes five cell therapies, four gene therapies and one gene-editing therapy. One of the cell therapies, Afami-cel, is an autologous T-cell receptor therapy, and would be the first T-cell therapy for a solid tumour indication (for Synovial Sarcoma). There are other products that could be approved, notably CT-053 by CARsgen Therapeutics, which recently had its New Drug Application (NDA) accepted by China’s NMPA15. Excitingly, Vertex and CRIPSR therapeutics have submitted their landmark approval to the FDA for exagamglogene autotemcel (exa-cel) to treat sickle-cell disease and beta-thalassemia. The regulatory agency is expected to decide within the next 8-12 months16.
Only 10 years after scientists have first shown human genome editing using CRISPR, the technology is rapidly moving in the clinical spotlight17. In the next blog article, we want to take a closer look at the emerging landscape of genome-editing as therapeutics and diagnostics.
3https://www.nature.com/articles/d41573-023-00050-8
5https://www.biopharmadive.com/news/hemophilia-gene-therapy-fda-approval-hemgenix-csl-uniqure/636999/
6https://www.fda.gov/vaccines-blood-biologics/carvykti
9https://investors.alnylam.com/press-release?id=26776
10https://www.iqvia.com/insights/the-iqvia-institute/reports/global-trends-in-r-and-d-2023
11IQVIA MIDAS QTR Dec 2022
Related solutions
Access new resources for advanced therapy development, from candidate identification through market authorization.
IQVIA is using vast quantities of data in powerful new ways. See how we can help you tap into information from past trials, patient reported outcomes and other sources to accelerate your research.
Learn how IQVIA is unlocking genomic data, at scale, while preserving privacy. Enabling a faster, more flexible, and less expensive approach for scientific enquiry into human biology, drug discovery and drug development.