Treating patients who have contracted COVID-19 has dominated the healthcare system’s attention and resources in the short term. However, the pandemic is likely to further strain the system. IQVIA is beginning to measure these effects in real world data (RWD), including health information that is captured in real-world settings, such as electronic health records, insurance claims, registries, and patient surveys.
While we have seen hospitals and clinics overwhelmed with the immediate need to treat patients with COVID-19, by examining IQVIA RWD sources, we are now beginning to measure and understand other downstream effects that the virus has had on managing health conditions.
Monitoring resource utilization
Throughout this pandemic, real world data will be essential to informing decisions and deepening our understanding of the real-world impact of COVID-19. This is addressed in-depth in an IQVIA white paper, “Leveraging Real World Data for COVID-19 Research: Challenges and Opportunities.”
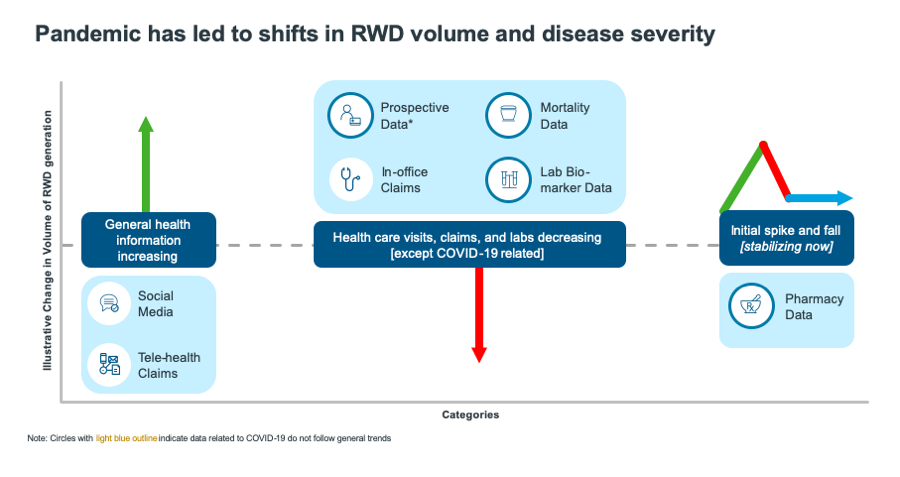
Already, we can see people’s use of healthcare resources reflected in RWD. Beginning in March 2020 in the U.S., we saw an immediate spike in pharmacy claims from IQVIA’s longitudinal prescription (LRx) claims database as patients stockpiled their medications. This spike was then followed by a stark drop, before leveling off as patients once again began to refill medications as instructed.
At the start of lockdown, we found that electronic medical records (EMRs) and insurance claims were capturing data on the most seriously ill patients, while less seriously ill patients reduced their visits to providers. This trend was measured by the decreased volume of claims data on in-office visits and laboratory biomarker data for non-COVID-19 related care. As a result, clinical information on these less severe patients must be found in data collected via insurance claims on telehealth visits, or via social listening, from wearables, and from reaching out directly to patients to measure treatments and outcomes.
One example of this direct-to-patient approach is reflected through IQVIA’s COVID-19 Active Research Experience (CARE) Project, in which individuals with COVID-19, or those at high risk of infection, are invited to provide information on their exposure. Their feedback enables researchers to understand more about the virus, and to connect people infected with COVID-19 to valuable resources and potential future studies.
Deepening reliance on RWD
The U.S. Food and Drug Administration (FDA) also sees tremendous value in using RWD to understand COVID-19 and to assess potential re-purposed treatments being used in practice. The Administration is relying on real world evidence (RWE), the evidence that comes from analyzing RWD, to inform policy decisions. Dr. Amy Abernethy, Principal Deputy Commissioner of Food and Drugs, explains that RWE is critical to our understanding of COVID-19 as she says, “Within the context of COVID-19, we’ve got this urgency to learn what we can...from the patients that are receiving care right now and trying to understand how [to] apply that as quickly as possible.”1
As an example, the COVID-19 Evidence Accelerator was launched in June 2020 by the Reagan-Udall Foundation for the FDA, in collaboration with Friends of Cancer Research and many other organizations, including IQVIA, to generate insights quickly from RWE and answer key questions about COVID-19 treatments and response. One of the catalysts for this effort came from a meeting in late March, during which the Observational Health Data Sciences and Informatics (OHDSI) network demonstrated the value of RWE to contribute to learning quickly about COVID-19 treatments and risks.
In just 88 hours, 350 OHDSI researchers completed eight observational studies on COVID-19. One examined the safety of hydroxychloroquine and azithromycin in rheumatoid arthritis patients, as the combination was being used as an experimental treatment for COVID-19. IQVIA’s Data Science team led the study group using three different IQVIA databases. They concluded that using the two products in combination was associated with an increased risk of cardiovascular events, and recommended caution around the use of this combination.
We are hopeful that this experience in using RWE will translate into a positive impact on guidelines for using RWE in approvals and labeling expansions in the future. The FDA is due to release new guidance on the use of RWE by the end of 2021, and it’s likely that the guidance will be influenced by the agency’s most recent experiences with RWE and COVID-19.
RWD may also relieve clinical trial site burden
Due to the pandemic, planning and recruitment for clinical trials has been disrupted, and many clinics have had to find creative ways to continue treating enrolled patients. This is an opportune time for sponsors to turn to RWD to relieve some of the burden on clinical trial sites. Suggestions include:
- Seeking all participants’ consent to link their clinical and real world data. By linking non-identified clinical trial data with pharmacy transactions, insurance claims, or EMRs, you can gain insights into treatment adherence and healthcare resource utilization.
- Considering secondary data, first. In some cases, the answer you need to expand an indication, for example, may be found in secondary data sources, precluding the need for a site-based study. In other cases, real-world cohorts might serve as external comparator arms.
- Moving to a direct-to-patient approach for collecting clinical trial data.
- Engaging with decision makers early and often. Regulators’ and payers’ evidence needs – and views of RWD are changing rapidly. Transparency and early collaboration can prevent unwelcome surprises and costly delays.
Other tips on reducing sites’ burden for caring for patients during the pandemic, can be found here.
IQVIA can help you analyze and understand the ever-expanding ecosystem of clinically-rich data in the context of your organization’s needs. By combining advanced analytics with unparalleled data and scientific expertise, IQVIA provides customers with innovative real world evidence approaches to meet stakeholder needs throughout the product lifecycle.
For a more in-depth discussion of the impact of COVID-19 on real-world solutions, listen to our webinar on the subject, or contact us directly to learn more.
1 Pink Sheet - Real-World Evidence On COVID-19: US FDA Approaching With 'Sense Of Urgency'