Discover intelligent insights from streamlined data and sophisticated analytics to transform trial workflows.
Clinical research teams aim to improve trial outcomes by deriving the right insights from the right data to shape the right actions. Critical to better decision making is understanding which “next best actions” to take in specific workflows based on information and insight.
The challenge begins with the proliferating volume, variety, and velocity of incoming data. After acquiring and integrating multiple sources of structured and unstructured data, sponsors require powerful tools to review the data, perform business intelligence and visualizations, prepare standardized data for regulatory submission, and more.
Many clinical and business intelligence applications promise “advanced analytics” and “intelligent insights.” But connecting data signals to actual workflows remains elusive because the insights are often confined to siloed systems. An insight generated within a particular application has limited value if it cannot be deployed to other solutions.
IQVIA’s approach is to deploy a central library of versatile, machine-augmented algorithms via APIs. Life science companies can utilize one specific algorithm in multiple applications to surface insights that drive actions across clinical workflows. Embedding that intelligence automates manual tasks and improves decision making.
Let’s discover some of IQVIA’s reusable algorithms for clinical environments.
Site Risk Analytics (SRA) algorithm
The SRA algorithm automates elements of site risk monitoring to support quality and risk management. SRA consolidates site intelligence data to provide clinical teams with deep insights about site availability and trends that impact data quality and compliance. This information can be used to adjust monitoring efforts.
This algorithm evaluates study data related to source data verification (SDV) backlogs and delivers a composite key risk indicator (KRI). In addition, the SRA algorithm calculates information related to COVID-19 such as site visit exception status, identified COVID-19 subject risk, related protocol deviation, and dates for the next remote and on-site visits. This information is weighted in relation to one another to help identify sites that are at greater risk or require mitigation and can be applied directly to consuming applications.
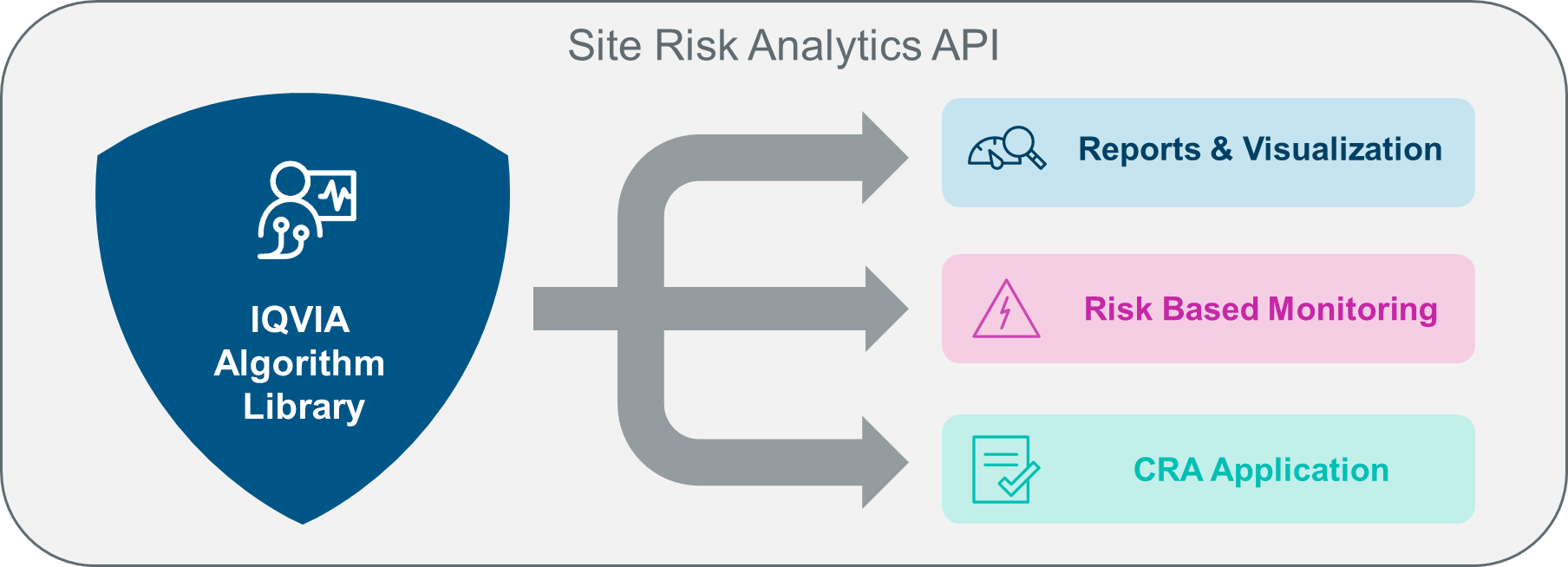
Customers may use the SRA algorithm not just for oversight reporting but to drive aspects of centralized and risk-based monitoring workflow solutions – delivering insights directly to the clinical research associate in CRA-specific apps.
Document Check (DC) algorithm
Another example of a reusable algorithm in IQVIA’s growing library is Document Check (DC). This algorithm assists trial master file (TMF) filing by confirming the quality of a scanned PDF document prior to submission to the trial master file. In concert with other algorithms, the DC algorithm can auto-categorize documents, saving time and preventing errors.
By making this algorithm available in the algorithm library, it can be reused across additional workflow solutions – such as an electronic investigator site file (eISF) application and in site portals by confirming the quality of a scanned PDF documents
An expanding library
Additional algorithms for clinical use cases include APIs for:
- Lab result and vital sign outliers, which move beyond minimum and maximum thresholds to identify outliers based on the clinical context of the patient
- Subject de-duplication, which compares subject records between sites to identify patients enrolled with different investigators
- Algorithms for trial design, patient recruitment and enrollment
Transform clinical outcomes
Sponsors can subscribe to algorithms that are appropriate for their clinical and operational needs. Using this algorithm library approach to build a consistent intelligence model also facilitates stakeholder collaboration. For example, to help Risk-based Monitoring Leads, Site Monitors, and Safety Leads respond to sites reporting protocol deviations or worrisome KRIs.
These advanced algorithms help power IQVIA’s Orchestrated Clinical Trial suites, including the new Clinical Data Analytics Suite (CDAS). CDAS offers a secure, scalable analytics ecosystem that embeds insights into workflows to accelerate data-driven decision making by stakeholders. Customers can also make Analytics as a Service (AaaS) a reality by applying algorithms to their own applications.
Having intelligence as a standalone capability is not enough. What matters is connecting timely and relevant insights to help stakeholders make smarter decisions about the workflows they’re responsible for. This model enables sponsors, sites, and patients to improve clinical outcomes such as faster development of new medications and safer patient experiences.
LEARN MORE
Related solutions
Ease the burden on your sites and make it easier and more appealing for patients to enroll and remain engaged.
Combine data science, technology, and analytics driven by artificial intelligence to support new efficiencies and business insights -- without additional capital investment.